Removal of Cr3+ From Tannery Wastewater Using Unmodified And Acid-Modified Arabica Coffee Husk Adsorbent
Keywords:
Arabica coffee husk, Biosorption, chromium (III), adsorption efficiency, TanneryAbstract
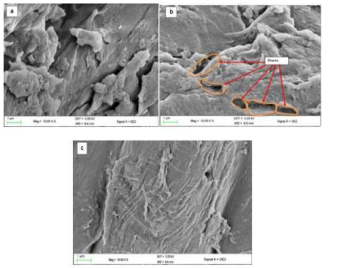
Tannery effluent containing chromium is a major problem in leather industry. In Kenya, tannery effluent is discharged directly into the main domestic sewage system, making wastewater treatment plants more difficult to operate. Chromium has long been employed in tanning because of the outstanding characteristics it provides to the leather as well as its ease of use. Only 60% of the entire chromium salt reacts with the hides, though. In other words, around 40% of the chromium remains in the solid and liquid wastes. Subsequently, the removal and reuse of the chromium content of these wastewaters is vital for environmental protection and economic reasons. This study explored the potential for removal and recovery of chromium from tannery effluent using unmodified (UCH) and modified (MCH) coffee husk biomass adsorbents. The raw coffee husk was subjected to sulphuric acid treatment, followed by characterization using FTIR and SEM analysis. The effects of initial metal ion concentration, agitation time, dosage, and pH were investigated in batch experiments. Effluent was obtained from Dogbones tannery in Dandora, Nairobi and was subjected to adsorption process at optimum conditions. Batch adsorption tests on these coffee husks revealed that as the initial metal ion concentration increased, the adsorption of metal ions increased as well. At pH = 4.5, the highest metal uptake was recorded. Maximum percentage removal was 47.52 % and 69.3 % for the UCH and MCH, respectively. For the UCH and MCH, the adsorption equilibrium was attained after 25 minutes and 15 minutes, respectively. Optimum dose of 3 g was realized for the two adsorbents. The presence of hydroxyl, carboxylic, and carbonyl functional groups was detected using FTIR. The surface texture and morphology of the biosorbent were revealed by scanning electron microscopy. The findings imply that coffee husk, in both modified and unmodified forms, is a low-cost, ecologically acceptable biosorbent that can be used to remove chromium ions from tannery effluent and other industrial effluents.
References
Kanamarlapudi, S.L.R.K., V.K. Chintalpudi, and S. Muddada. Application of Biosorption for Removal of Heavy Metals from Wastewater. Journal of IntechOpen . pp 80-101, 2018
Jawad, A.H., A.S. Abdulhameed, and M.S. Mastuli. Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. Journal of Taibah University for Science, Vol 14, issue 1, pp. 305-313, 2020
Aziri, S., S. Meziane, and N. Berkane. Biosorption of chromium (VI) from aqueous solution by seed powder of prickly pear (Opuntia ficus indica L.) fruits. Journal Separation Science and Technology, Volume 55, issue 14, pp. 2459-2469, 2019.
El-Naggar, Ragaa A. H; Ibrahim E. M; Marwa S. A. H and Nashwa H. R. Biosorption optimization, characterization, immobilization and application of Gelidium amansii biomass for complete Pb2+ removal from aqueous solutions. Journal of Chemosphere, Vol 8, issue 1, pp. 13456, 2018.
Aranda-Garcia, E., Lilian M. B; Gabriela P. C and Eliseo C. U. Effect of pH, ionic strength, and background electrolytes on Cr(VI) and total chromium removal by acorn shell of Quercus crassipes Humb. & Bonpl. Journal of Springer International Publishing Switzerland, Vol 186, issue 10, pp. 6207-6214, 2014
Huang, K., Y. Xiu, and H. Zhu. Removal of hexavalent chromium from aqueous solution by crosslinked mangosteen peel biosorbent. International Journal of Environmental Science and Technology, Vol 12, issue 8, pp. 2485-2492, 2014.
Sahmoune, M.N., K. Louhab, and A. Boukhiar. Advanced biosorbents materials for removal of chromium from water and wastewaters. Journal of Environmental Progress & Sustainable Energy, Vol 30, issue 3, pp. 284-293, 2011
Kurniawan, A. T; Gilbert Y. S. C; Wai-hung. L; Sandhya B. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Science of Total Environment Journal,. Vol 366, issue 2-3, pp. 409-26, 2006
Alemu, A., Brook L; Nigus G. Adsorption of chromium (III) from aqueous solution using vesicular basalt rock. Cogent Environmental Science, Vol 5, issue 1, 2019.
Atieh, M.A., Omer Y B; Bassam S. T; Alaadin A. B; Mazen K; Mamdouh A; Mohamed F and Faraj A. A. Removal of Chromium (III) from Water by Using Modified and Nonmodified Carbon Nanotubes. Journal of Nanomaterials, pp 1-9, 2010.
Netzahuatl-Munoz, A.R., C. Cristiani-Urbina Mdel, and E. Cristiani-Urbina. Chromium Biosorption from Cr(VI) Aqueous Solutions by Cupressus lusitanica Bark: Kinetics, Equilibrium and Thermodynamic Studies. Publishing Library of Science Journal, Vol 10, issue 9, pp. 1 - 23, 2015.
Nthiga E. W; Jane M; Ahmed H. and Ruth W. Efficacy of Adsorption of Cu (II), Pb (II) and Cd (II) Ions onto Acid Activated Watermelon Peels Biomass from Water. International Journal of Science and Research (IJSR), Vol 5, issue 8, pp. 671-679, 2016.
Eneida Sala Cossich, C.R.G.T., Teresa Massako Kakuta Ravagnani. Biosorption of chromium (III) by Sargassum sp. biomass. Electronic Journal of Biotechnology. Vol 5 issue 2, pp. 8. 2002.
Nthiga, E.W., Jane Murungi, Ruth Wanjau, Ahmed Hassanali. Application of chemically modified avocado seed for removal of Copper (II), Lead (II) and Cadmium (II) ions from aqueous solutions. International Journal of Research in Engineering and Applied Sciences, Vol 6, issue 8, pp. 15, 2016.
Villabona-Ortiz, A., Candelaria T. T; Adriana P. H and Angela D. G. Preparation of biosorbents from corn residual biomass and its application for Cr (VI) uptake. Journal of Contemporary Engineering Sciences, Vol 11, issue 29, pp. 1401-1409, 2018.
Díaz-Muñoz, L.L., Bonilla A; Reynel H. E; Mendoza D. I. Sorption of heavy metal ions from aqueous solution using acid-treated avocado kernel seeds and its FTIR spectroscopy characterization. Journal of Molecular Liquids, Vol 215 pp. 555-564, 2016.
Nikhil C. T and Bhalerao S.A. Biosorption of Chromium (VI) from aqueous solution using unmodified and nitrilotriacetic acid modified leaves of Ficus benghalensis L. International Journal of Recent Scientific Research, Vol 10, issue 02, pp 31045-31053, 2019.
Nur-E-Alam, M., Abu S. M, Farid A and Mafizur R. Adsorption of chromium (Cr) from tannery wastewater using low-cost spent tea leaves adsorbent. Journal of Applied Water Science, Vol 8, issue 5. pp 34-67, 2018.
Rezaei, H. Biosorption of chromium by using Spirulina sp. Arabian Journal of Chemistry, Vol 9, issue 6, pp. 846-853, 2016.
Ko?ody?ska, D., J. Krukowska, and P. Thomas. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Journal of Chemical Engineering, vol 307, pp. 353-363, 2017.
Gonultas, O. and Z. Candan. Chemical characterization and ftir spectroscopy of thermally compressed eucalyptus wood panels. Maderas. Ciencia y tecnología, (ahead): Vol 20, issue 3, 431-442, 2018.
Godlewska, K., K. Marycz, and I. Michalak. Freshwater green macroalgae as a biosorbent of Cr(III) ions. Journal of Open Chemistry, Vol 16, issue 1, pp. 689-701, 2018.
Samadi, N., R. Hasanzadeh, and M. Rasad. Adsorption isotherms, kinetic, and desorption studies on removal of toxic metal ions from aqueous solutions by polymeric adsorbent. Journal of Applied Polymer Science. pp 1 - 13, 2014.
Cheruiyot, G.K., Wycliffe C. W; Joyce K and Esther M. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Journal of Scientific African, Vol 5, issue 2-10, 2019.
Pérez A. Aguilar M. I; Meseguer V. F and Juan F. O. S, A.B. Biosorption of chromium (III) by orange (Citrus cinensis) waste: Batch and continuous studies. Chemical Engineering Journal, vol 155, issue 1-2, pp. 199-206, 2009.
Ndung`u S. N., W.R.N., Nthiga E.W., Ndiritu J., Mbugua G. W. Complexation equilibrium studies of Cu2+, Cd2+ and Pb2+ ions onto ethylenediamine quaternised Artocarpus heterophyllus L. seeds from aqueous solution. Journal of Applied Chemistry, Vol 13, issue 12, pp. 1-12, 2020.
Nthiga, E.W., Ndung`u, Samuel Ng`ang`a, Kibet, Kelvin, Wanjau, Ruth Nduta. Removal of Cr3+ ions from a model solution by HCL treated Artocarpus heterophyllus L. seeds: Equilibrium and Kinetic Study Inernational Journal of Research and Innovation in Applied Science, Vol 6, issue 2, pp 38-44, 2021.
Shafqat, F., Haq N. B; Muhammad A. H and Ammara Z.. Kinetic and Equilibrium Studies of Cr(III) and Cr(VI) sorption from aqueous solution using rosa gruss an teplitz (Red Rose) waste biomass. Journal of Chilean. Chemical Society, Vol 53, issue 4. pp. 6, 2008.
Kariuki, Z., Kiptoo, J., Onyancha, D. Biosorption studies of lead and copper using rogers mushroom biomass `Lepiota hystrix`. South African Journal of Chemical Engineering. Vol 23, pp 62 - 70, 2009.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it, under the condition that the authors are given credit and that in the event of reuse or distribution, the terms of this license are made clear.





