Repercussion of Routine Laboratory Practice in the Determination of Degree of Dissociation of Anion of Weak Electrolyte Using Corelation of Nernst Equation with Equivalent Conductance at Equilibrium
Keywords:
Electrolyte, Dissociation, ElectrostaticAbstract
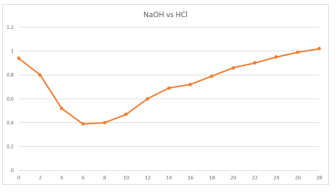
Strong electrolytes are totally ionized at all dilutions; hence the Ostwald`s dilution law only applies to weak electrolytes and completely fails in the case of strong electrolytes. Water molecules surround and solvate the ions, weakening the strong electrostatic interactions between them, which causes the ions in the solid to separate and scatter equally throughout the solution when ionic substances dissolve in water. Under normal circumstances, ionic compounds will dissociate almost entirely when dissolved, which is why they are categorised as strong electrolytes. This process represents a physical change known as dissociation.1,2. In other words, when dissolved in water, they do not produce a lot of ions. They create a tiny current with their solutions. As the ions transition from their fixed and ordered positions in the crystal to the mobile and considerably more disordered states in solution, the system becomes more chaotic due to the loss of electrostatic attraction, which allows each hydrated ion in a dilute solution to move independently. Sometimes, the electrostatic interactions between the ions and water molecules are too weak, causing the crystal to become intractable because the increase in disorder cannot offset the energy needed to separate the ions. 3,4. That is, they produce relatively few ions when dissolved in water. Their solutions produce a small current. Therefore, unlike in a perfect solution, ions are not evenly distributed throughout the solution. In present research paper the study of deviation from the ideal behavior has been proposed through experimental outcomes.
References
K.J. Laidler, J.H. Meiser, “Physical Chemistry,” Benjamin-Cummings Publishing Co., pp. 259, 1982
C.H. Langford, R.A. Beebe, “The Development of Chemical Principles” Courier Corporation, pp. 135, 1995
K.J. Laidler, :Physical chemistry with biological applications” Benjamin-Cummings Publishing Co., pp. 266, 1978
“Best test preparation for the GRE Graduate Record Examination Chemistry Test,” The Research and Education Association, pp. 149, 2000
P. Atkins, J. de Paula, “Physical Chemistry,” Oxford University Press, pp. 762, 2006
Conductivity, IUPAC Gold Book.
K.J. Laidler, J.H. Meiser, “Physical Chemistry,” Benjamin-Cummings Publishing Co., pp. 281–283, 1982
K.J. Laidler, J.H. Meiser, “Physical Chemistry,” Benjamin-Cummings Publishing Co., pp. 256, 1982
P. Atkins, “The Elements of Physical Chemistry,” Oxford University Press, 2001
G. W. Castellan, “Physical Chemistry,” Benjamin-Cummings Publishing Co., 1983
K.J. Laidler, J.H. Meiser, “Physical Chemistry,” Benjamin-Cummings Publishing Co., pp. 273, 1982
P. Atkins, J. de Paula, “Physical Chemistry,” Oxford University Press, pp. 766, 2006
Y.C. Wu, P. A. Berezansky, “Low Electrolytic Conductivity Standards,” J. Res. Natl. Inst. Stand. Technol, 1995
A.V. Pandya Synthesis, Conducting Properties and Applications of Poly Ethyl Aniline, Pandya International Journal of Scientific Research in Chemical Science, Volume-3 , Issue-5 , Oct 2016, ISSN 2455-3174
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Anosh Charles

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it, under the condition that the authors are given credit and that in the event of reuse or distribution, the terms of this license are made clear.





