Preparation of Beta-Diketone Complex under Potentiometric Study with Fe (II) and Co (II) Transition Metal Ions
Keywords:
Potentiometric Study, bita–Diketone, Metal ions, pH and Stability ConstantAbstract
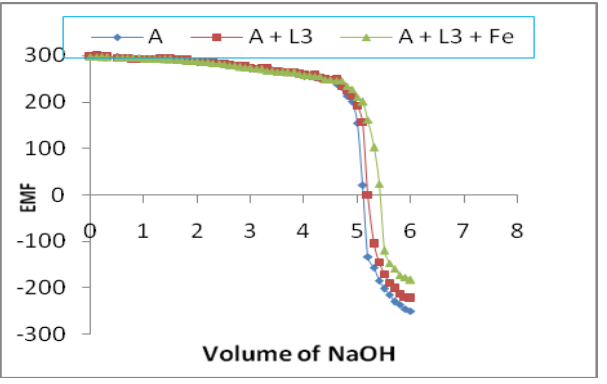
Potentiometric study of bita–diketone complexes of iron, cobalt was carried out in aqueous media by titrating with standard NaOH solution. The complexes formation between Fe(II) and Co(II) metal ions and 1-(5-methoxythiophen-2-yl)-3-(2,4- dihydroxyphenyl)propane-1,3-dione (L) have been studied at 0.1 M ionic Strength at room temperature. It is observed that iron, cobalt, nickel and copper metal ions formed 1:1 and 1:2 complexes with ligands (L). The obtained data were used to compare the values of pH and metal-ligand stability constants.
References
Cullen W. R., Wickenheiser E. B., Rhodium(I) complexes of ?-diketonates and related ligands as hydrosilylation catalysts, J. Organomet. Chem., 370, 141, 1989.
Lewis F. D., Miller A. M., Salvi D. G., Spectroscopy and photochemistry of Nickel(II), Palladium(II), and Platinum(II) ?-diketonates, Inorg. Chem., 34, 3173, 1995.
Haltiwanger C., Wenzel T. J., Williams E. J., Seivers R. E., Studies of metalchelates with the novel ligand 2,2,7-trimethyl-3,5-octanedione,Polyhedron , 4(3), 369, 1985.
Aho P., Backstrom R., Honkanen E., Linden I., Nissien E., Pohto P., nr 163044B1, PL Patent, 1994.
Miyamato M, Murata T., Yokotani H., nr 3708504, US Patent, 1973.
Aho P., Backstrom R., Koponen A., Linden T., Lonnberg K., Lotta T.,Pippuri A., Pohto P., nr 745338, AU Patent, 2002.
Odunola O., Woods J., Synthesis, Electronic and Magnetic properties of some 3-substituted-2,4-pentanedioneato oxovanadium(IV) complexes and their methylpyridine adducts, Synth. React. Inorg.Met. Org. Chem., 31(7),1297, 2001.
Anderson R. A., Kiefer R. L., Kim M., Thiebeault S. A., Modified polymeric materials for durability in the atomic oxygen space environment[J]. Nucl.Instr.And Meth. Phys. Res.,B208,300, 2003.
Schubert U., Silica-based and transition metal-based inorganic-organic hybrid materials-a comparison. J. Sol-Gel Sci. Techn.,26,47, 2003.
Meshkowa S. B., The dependence of the luminescence intensity of lanthanide complexes with ?-diketone on the ligand form. Journal of Fluorescence, 10(4),333, 2000.
Pietraszuk C., Staniszewski B., Witt K., Urbaniak W., Polskiezg?oszeniepatentowe P-393338, 2010.
Urbaniak W., nr 188732, Patent RP, 2004..
Staniszewski B., Urbaniak W., A simple and efficient synthesis of 3-substituted derivatives of pentane-2, 4-dione, Chem. Papers,63(2),212, 2009.
Irving H. M. and Rossotti H.S., J. Chem. Soc. 2, 904, 1954.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it, under the condition that the authors are given credit and that in the event of reuse or distribution, the terms of this license are made clear.





